eConsent is rapidly becoming the new consent standard for trials
Electronic Informed Consent, also called eConsent, is rapidly becoming the new consent standard for clinical trials. The global COVID-19 pandemic sped up the trend, with eConsent ensuring informed consent continuity amid impossible or avoided face-to-face contact. Trial participants who feel insecure or inconvenient about visiting the site can consent from home.
Reap the benefits of eConsent
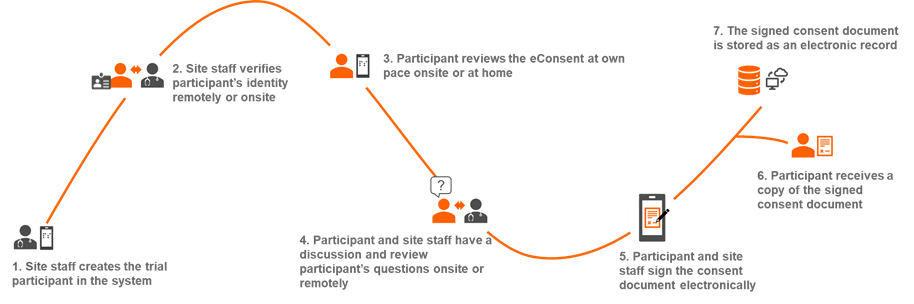
eConsent can improve one of the most important steps in the consenting process – the process of informing the participant adequately. eConsent can provide options such as interactive learning using multimedia components, a tiered approach to tailor to learning preferences, testing of the participant’s comprehension, and review of the participant’s questions. Furthermore, it can allow for continuous improvement of the consent content through surveys and rapid notifications to the participants of any amendments. Trial participants can review the eConsent at his/her own pace at home before the discussion with the site staff. This can overall improve the discussion, engage the participant, and increase protocol adherence and enrollment rates.
Ensuring the integrity of the consent process with eConsent involves reducing consent-related findings, achieving global signature compliance, providing reliable date/time stamps, and facilitating easy access to audit trails.
Increased tracking capabilities can enable the ability to get real-time consent status across sites and immediately identify any consenting issues.
By enabling remote reconsenting and facilitating the rapid delivery of new information, eConsent can enhance the reconsenting process for trial participants.
Considerations
It is important to consider whether eConsent would be the right fit for your trials as early as possible as it can take several months to set up eConsent from vendor selection to first participant enrollment. It can be a good idea to run a pilot trial before implementing eConsent across the entire organization.
The organizational impact is important to address as well as site engagement. The impact on sites and areas of resistance can be identified as well as methods to be implemented to overcome these. The requirements for IRB/IEC submissions should be fully understood and may be different for each country. Ways to improve the efficiency of the submission approval process should be identified.
Selecting and implementing eConsent
It is important to carefully analyze and plan your eConsent implementation. HERAX offers assistance throughout all project phases, including early analysis, business case development, vendor selection, trial planning, and impact analysis. Leveraging our experience and market knowledge, we meticulously assess solutions through a structured vendor selection process.
Experts at HERAX specialize in creating change management strategies crucial for successfully adapting eConsent within the organization and trial teams.
After a thorough planning phase, we can help execute the plan, by establishing the new business processes and technology and training of trial managers and monitors. We are experts in validation and can also perform go-live activities including establishing operational procedures.
Are you considering eConsent for your clinical trials?
We can assist in initiating your eConsent analysis, vendor analysis, business case, or pilot to evaluate if eConsent is the optimal solution for your trials. A thorough analysis of the business needs is essential to avoid unnecessary costs and changes.
We can assist you by combining a proven Life Science specific project methodology with a best-in-class knowledge of the trial execution area.
Visit our LinkedIn Page here.

Sarah Sendrup
Chief Compliance Officer and Principal Consultant
Sarah has supported clients from the pharmaceutical and medical device industry, both in Denmark and the rest of Europe. As a senior consultant, she specializes in IT Quality, IT project management, and business processes for computerized systems in Life Science. She has a deep knowledge of the business process supported by CTMS, EDMS, eTMF, and RIMS and a solid understanding of the regulatory requirements. She is also one of our specialists in eConsent solutions. Sarah has a strong background in pharmaceutical research, which she has been applying to her projects over the past years. Sarah holds a Master of Science in Engineering in Biotechnology. She has worked in both research and biopharmaceutical production before joining HERAX.
